For general enquiries please contact us on
+44 1223 248888
enquiries2026@innoviatech.com
St Andrew's House, St Andrew's Road
Cambridge CB4 1DL United Kingdom
FIND US
For general enquiries please contact us on
+44 1223 248888
enquiries2026@innoviatech.com
St Andrew's House, St Andrew's Road
Cambridge CB4 1DL United Kingdom
FIND US
Lubrication chemistry underpins everything from engines to suncreams, making it a brilliant example of the value of innovating across the sectors.
What connects tiny watch components to gigantic mining machines, aching joints to deep-sea fish, and sizzling frying pans to sexual health? Lubrication.
You engage with lubrication hundreds of times every single day without even thinking about it.
In my three years at Innovia, lubrication has come up more often than I—a chemist, not an engineer—ever expected. In this article I explore the fundamentals of lubricants and why we use them. Although lubricants are found in a wide variety of different contexts, Looking with a cross-industry lens can reveal these parallels and spark fresh insights.

Friction is shockingly expensive – the GDP saving from tribology improvements is estimated to be 1–2% of GDP [2]. Globally this is more than $1 trillion dollars!
And that is without even mentioning the sustainability benefits: roughly 23% of total global energy consumption is used in frictional contacts, 20% on friction and 3% to replace worn parts [1].
Clearly, when used effectively, lubrication has a dramatic positive impact on both the bottom line and the planet.
Tribology can get complicated. Like, really complicated. Before we try to grapple with thixotropic graphene oxide nanoparticle Pickering emulsion lubricants [3], let’s cover the basics.
When two surfaces rub against one another, friction is the force opposing their movement. In dry contacts, this force typically stems from asperities, or microscopic surface roughness. These rugged projections interlock, becoming sites of friction and wear.
Putting a liquid (lubricant) in between these surfaces reduces friction. This liquid pushes the surfaces apart, reducing asperity contact. As the layer gets thicker, eventually, the surfaces don’t contact at all, and motion is only opposed by fluid flow. The different regimes of lubricated friction are described by the Stribeck Curve (Figure 1).
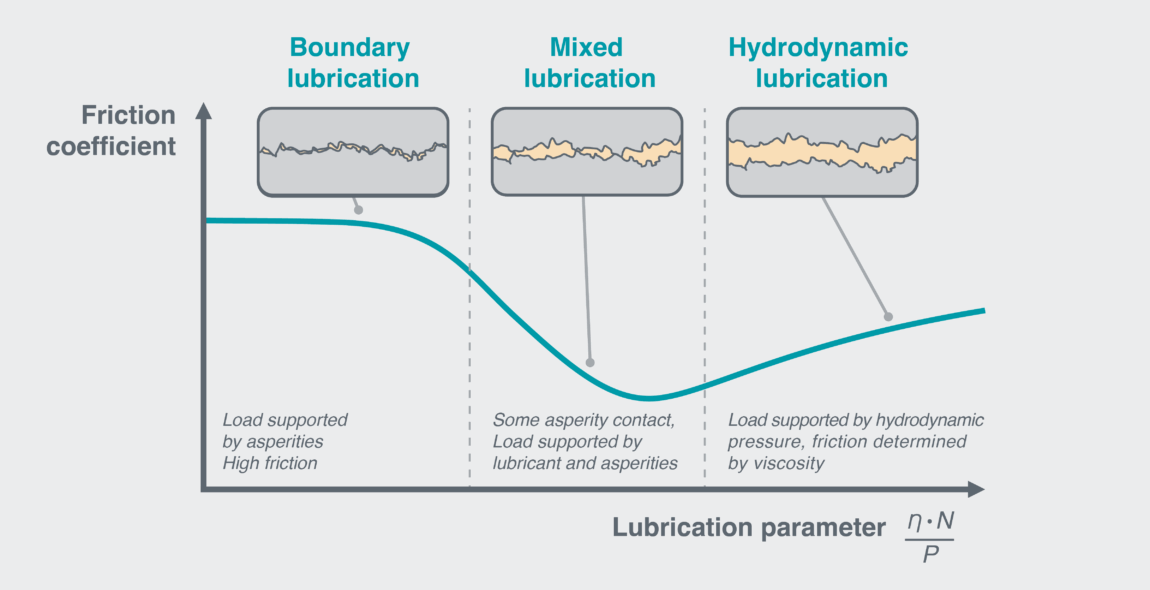
Figure 1: The Stribeck curve, showing three regimes of lubricated friction. The lubrication parameter is a dimensionless quantity equal to the fluid’s dynamic viscosity η, multiplied by the velocity N, divided by the applied load per unit length P. Therefore, you move further right along the curve for higher viscosities, greater speeds, or smaller loads. Note, this parameter is often shown as lubricant thickness over asperity height. Friction is minimised at the point where asperity contact disappears, at the beginning of the hydrodynamic regime.
, so the Stribeck curve is useful to know what regime your system is in.
Despite having a huge variety of applications, to a chemist like me, a lot of lubricants end up looking pretty similar.
‘Traditional’ lubricants are composed of medium-length, relatively inert, non-polar chains of carbon atoms. Let’s consider these terms to understand why some desirable properties appear.
The description above covers most petroleum base oils, such as those used in cars. If we then allow some chemical groups on the ends or sides of these carbon chains, we can play with properties such as viscosity, surface adhesion, boiling point, reactivity, and biodegradability.
This chains + side groups description covers lubricants ranging from vegetable oils in frying pans to lithium greases in car wheel bearings, from polyalphaolefins in wind turbines to natural olefins in skincare. From this simple description alone, we have encompassed the majority of industrially useful lubricants (Figure 2)!
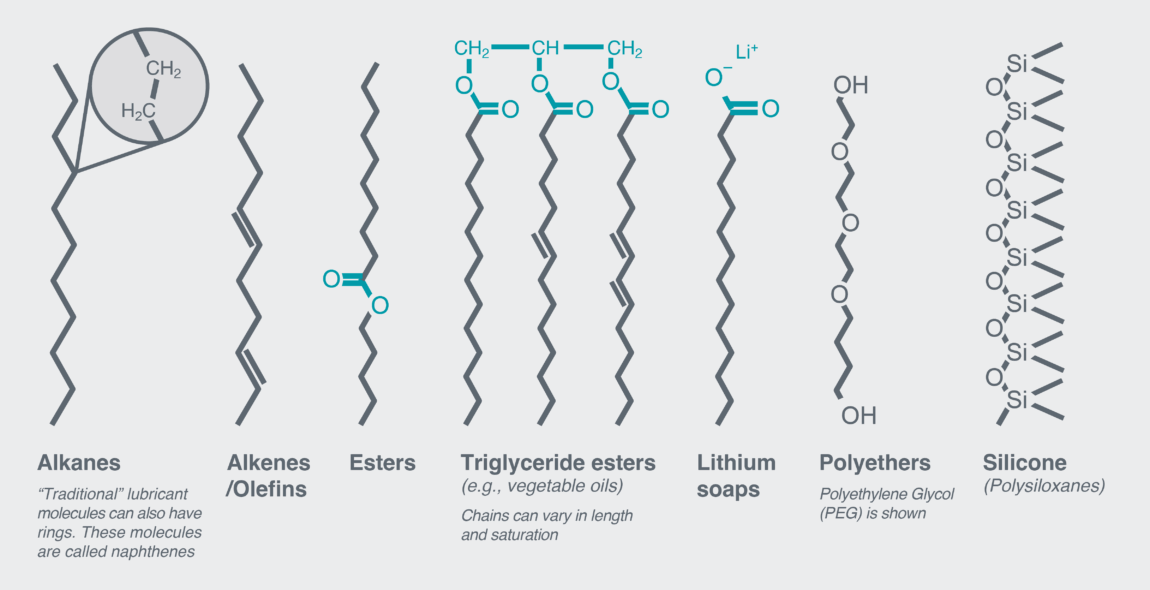
Figure 2: A selection of common lubricant molecule classes. This illustrates how common this non-polar chain of atoms is.
Of course, due to the diversity of lubricant applications, there are stranger chemistries: some lubricant’s chains obey the same principles but are made of other atoms. For example, polyethers (carbon-oxygen) and silicones (silicon-oxygen) in high temperature and personal health applications.
In applications where hydrophilic behaviour, biocompatibility, or water solubility are important, similar chains can be used to thicken water into gels. This is seen with polyethers, as well as proteins (such as the mucins in your saliva), and is common in biological systems, medical applications, and consumer health.
People are always surprised to learn that, on a fundamental level, the oils which make food not stick to pans, or creams apply smoothly to skin, are only a few atoms away from those which help run cars or wind turbines. Given the underlying commonalities in physics and chemistry, I hope you are beginning to see why this cross-industry experience can provide huge value when innovating.
Now let’s look at some of the links between different lubricant applications. There are truly thousands of possible examples here, so this is just a glimpse.
4.1 Conducting and withstanding heat
Temperature is an important factor for lubricants – hot liquids are less viscous and can oxidise or evaporate; cold liquids thicken and freeze.
In cutting and metalworking, lubricants reduce friction and heat generation but simultaneously act as a coolant. High thermal capacity and conduction wicks heat away from cutting points, preventing reactions and damage to parts.
Conversely, in frying, oil conducts heat to the food surface, while avoiding sticking and degradation. In both systems, high thermal stability is key and is achieved in broadly the same ways.
When searing food, we can choose high smoke point oils like avocado with longer, more saturated carbon chains. These contain natural (or added) antioxidant molecules, such as tocopherols (Vitamin E) or phenolics. It’s also noteworthy that many high temperature cooking oils have heat-sensitive components removed, such as the milk solids removed from butter to make ghee.
In just the same way, oil-based cutting fluids use thermally stable saturated carbon chains, with the heat-sensitive species filtered out. Synthetic antioxidants are then added, sometimes even the same molecules as in frying oils!
4.2 Getting lubricants in the right places
Different systems apply lubricants in different ways. Some need to apply lubricants consistently over time, but without regularly dismantling the entire system.
In mechanical systems, drip-feeding devices can slowly feed lubricants into points of friction, controlling how much is added. Clever, right? But, as is often the case, biology already beat us to it.
The viscous synovial fluid between our joints is a natural lubricant which helps us move. Synovial fluid forms a thin (~50 µm) layer, but this layer must be maintained as load is put on a joint. Cleverly, when weight is applied, more of this fluid is squeezed out of sponge-like cartilage in a process called “weeping lubrication” [4]. Bio-inspired weeping lubricant joints are even being considered for articulating robotics [6].
Even more incredibly, this movement of synovial fluid plays a secondary role as transport for waste and nutrients into and out of cartilage. In much the same way, the oil filter in your car removes contaminants which build up in engine oil. Natural systems can be a huge inspiration for analogous mechanical ones.
4.3 Optimising viscosity: through thick and thin
We’ve seen that viscosity is a key property of lubricants, determining the regime and degree of lubrication. If it’s too thick, your lubricant cannot flow and is worse at reducing friction; too thin, and the lubricant ends up where you don’t want it.
Formulators will often tune viscosity with additives, and these too can also be enhanced through cross-industry thinking. For example, the principles involved in thickening a mineral oil to make a lubricating grease are the same as those in structuring a vegetable oil in a food system.
If you’d like to see more of this in action, check out how Innovia used these fundamentals to help Shell’s Lubricant Discovery Hub to create new-to-world thickeners for their lithium soap greases [link].
4.4 Sticking and slipping
Counterintuitively, for substances which reduce friction, ‘stickiness’, on a molecular level, can be extremely beneficial to lubricants.
‘Sticky’ lubricant molecules form thin films that spread to fully cover surfaces, protecting against scratches and corrosion while keeping the lubricant where it’s needed. Crucially, in extreme conditions, when surfaces get very close to one another, this layer can dramatically reduce the friction involved.
We see these ‘sticky’ molecules in the smallest systems, such as in anti-friction coatings and nanotechnology; single layers of molecules chemically bound onto a surface generate potent lubricants [5]. But this is also key for much larger systems. Common metal-metal lubricants like esters and lithium greases function on a similar principle.
For another example, we can think about cast-iron pans. Food molecules, especially proteins, like to form complexes with heated metal surfaces and stick. To avoid this, when you buy a new cast iron pan, you must ‘season’ it to make it non-stick, through oiling and heating steps. The heat polymerises lubricating oil molecules together, forming a non-stick water-repellent film bound to the metal surface. This strong layer is non-stick in two ways – it fills in asperities and blocks food from bonding to the metal.
Sometimes, instead of choosing molecules which stick to surfaces, we modify surfaces to hold lubricants in place, such as in intricate lubricated watch mechanisms. These are pre-treated with a chemical called an epilame, which tunes the surface attraction, keeping lubricants exactly where we want them.
Even car windshields have polymerised, surface-bound molecules made of silicon oxides. These lubricate and repel water droplets, causing rain to bead up and roll off another ‘sticky’ lubricant, analogous to pans and watches!
4.5 Protecting the human body
Lubricants are just as important in medical and consumer health applications. Of course, these bring additional challenges such as biocompatibility, as well as how consumers perceive textures.
Often in these applications, lubrication is being used to avoid abrasive damage to soft tissues and allow even application, whether that is during sex, when shaving, or while applying creams, oils, cosmetics, or eye drops. Careful consideration of tribology and formulation is needed to give a pleasing, smooth sensation and balance consumer perceptions of textures of a product being greasy, sticky, slippery, or soft.
These factors not only drive purchasing of products, but they also maintain health, for example by ensuring an effective and easy coverage of suncream or topical medications.
I hope I’ve made the case effectively that lubrication is everywhere. Lubricants are diverse, important, and span a wide array of industries. But, at a fundamental level, they can look pretty similar. Obviously, I’m biased, but I do think lubrication is a brilliant example of how, when you’re in a highly specialised industry, there is value in taking a broader view. Looking from a cross-industry lens can give you that fresh perspective.
Do you have a problem where the obvious solution just isn’t sticking? Does making one change send everything else out of balance? Or do you just wish that you could get things running more smoothly?
Whatever the challenge, we’d love to talk about it, particularly if it’s sticky, slippery, or both!
Contact: Jonathan Shaw at lubrication@innoviatech.com
Jonathan Shaw: LinkedIn
References:
1. Holmberg, Kenneth, and Ali Erdemir. “Influence of tribology on global energy consumption, costs and emissions.” Friction 5 (2017): 263-284.
2. Woydt, M. “The importance of tribology for reducing CO2 emissions and for sustainability.” Wear 474 (2021): 203768.
3. Yu, Weiyan, et al. “Versatile Pickering Emulsion Gel Lubricants Stabilized by Cooperative Interfacial Graphene Oxide-Polymer Assemblies.” Materials Horizons (2024).
4. Gebeshuber, Ille C., and George Van Aken. “Friction and Lubricants Related to Human Bodies.” Lubricants 5.1 (2017): 4
5. https://www.gelest.com/applications/case-coatings-adhesives-sealants-and-elastomers/self-assembled-monolayers/
6. Miki, Akihiro, et al. “Designing Fluid-Exuding Cartilage for Biomimetic Robots Mimicking Human Joint Lubrication Function.” 2024 IEEE 7th International Conference on Soft Robotics (RoboSoft). IEEE, 2024.

Jonathan is an Innovation Consultant at Innovia Technology with a background in chemistry and materials science. He specialises in tackling complex challenges across food, FMCG, and sustainability, particularly those with no clear solution and seemingly contradictory requirements. From firefighter’s outfits to natural colours to electric vehicles, one thread that ties much of his work together is figuring out how to replace the chemicals in a system without compromising on the system’s properties.
His favourite projects are those requiring a multi-disciplinary approach. The best innovation emerges when working with teams of highly skilled engineers, behavioural scientists, designers, biologists, and business strategists. He is passionate about translating science and technology into tangible and meaningful benefits for consumers and solving problems to make the world a better place.
Contact: Jonathan Shaw at lubrication@innoviatech.com
Jonathan Shaw: LinkedIn